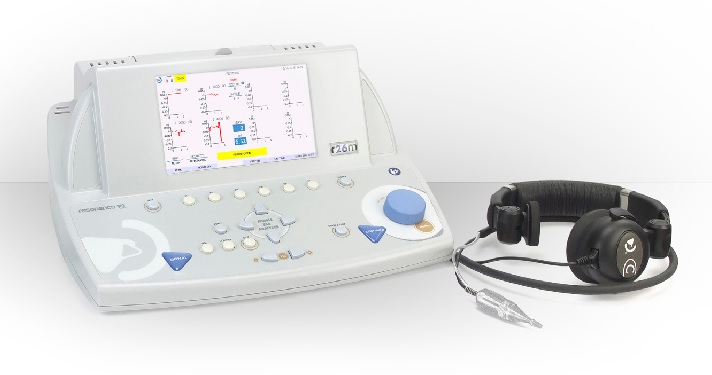
Description
GENERAL SPECIFICATIONS
POWER
DIAGNOSTIC MIDDLE EAR ANALYZER
DIMENSIONS AND WEIGHT
- L x W x H: 370x290x180 mm
- Net weight: 3.5 kg
- Body material: Bayblend® self-extinguishing
TEST TYPES
Tympanometry, Acoustic Reflex, Reflex Decay, Quick Test, Quick Screening
DISPLAY
- 7” TFT Color display
USER INTERFACE
- Multilingual
PRINTER
- Built-in fast thermal printer with paper width: 112 mm supplied as standard part
REPORTS
- Printed on thermal printer
- .pdf report create directly from the device and stored on USB Pen drive with
possibility to add patient data and tests comments via the USB Keyboard (optional)
- Data transfer to PC using Resonance Management Data Suite
“CHILDREN” FEATURE
- To help keep the child distracted while running screening “Quick Check” a series of
animated images appears on the color display
DATA TRANSFER TO PC
- Via cable trough USB port
COMMUNICATION PORT
- Nr.1 USB host type A
- Nr.1 USB slave type B
WINDOWS® COMPATIBLE SOFTWARE
- Resonance MDS Management Data Suite
POWER SUPPLY
- 110-240V AC 50/60Hz 40VA
- Fuses: 2 x T 1 A L 250V
CONSUMPTION
- Max current: 0,15A
- Power consumption: 40VA
ENVIRONMENTAL
OPERATING ENVIRONMENT
- Storage: -20° C up to +50° C
- Operating: +15° C up to +35° C
- Humidity: up to 90%, (non condensing)
- Ambient pressure: from 700hPa up to 1060hPa
COMPLIANCE/REGULATORY STANDARDS
Designed, tested and manufactured to meet the
European and International Standards:
- MDD 93/42/EEC and update 2007/47/CEE: Class IIa (as referred to in Annex IX, rule 10 of said MDD 93/42 EEC )
- Safety: IEC 60601-1, 3rd and 2nd edition, Class 1 Type B
- EMC: IEC 60601-1-2
- Impedance: IEC 60645-5/ANSI S.3.39 Type 2
QUALITY SYSTEM
Manufactured, designed, developed and marketed under an ISO 13485, ISO 9001 certified quality system.
Medical CE marks and FDA approval









Reviews
There are no reviews yet.